嘌呤霉素Puromycin 嘌呤霉素盐酸盐溶液(10mg/mL)|CAS 58-58-2
产品说明书
FAQ
COA
已发表文献
嘌呤霉素(Puromycin)是由白黑链霉菌(Streptomyces alboniger)发酵代谢产生的一种氨基糖苷类抗生素,通过抑制蛋白质合成而杀死革兰氏阳性菌,各种动物和昆虫细胞。某种特殊情况下有效作用大肠杆菌。作用机制在于嘌呤霉素是氨酰-tRNA分子3´末端的类似物,能够与核糖体的A位点结合并掺入到延伸的肽链中。嘌呤霉素同A位点结合后,不会参与随后的任何反应,从而导致蛋白质合成的提前终止并释放出C-末端含有嘌呤霉素的不成熟多肽。
嘌呤霉素产生菌Streptomyces alboniger内发现的pac基因编码嘌呤霉素N-乙酰转移酶(PAC),赋予机体对嘌呤霉素产生抗性。这一特性如今普遍应用于筛选特定携带pac基因质粒的哺乳动物稳定转染细胞株。
嘌呤霉素在细胞稳转株筛选中的普遍应用与慢病毒载体的特性有关,现在商业化的慢病毒载体多数都携带pac基因。在某些特定情况下,嘌呤霉素亦可以用来筛选转化携带pac基因质粒的大肠杆菌菌株。
本品是无菌的、溶于蒸馏水的嘌呤霉素盐酸盐溶液,浓度为10 mg/mL(10 mg/mL in H2O),可直接用培养基或其他缓冲溶液稀释使用,适用于细胞培养,常用工作浓度为1~10 µg/mL。
产品信息
|
货号 |
60209ES10/60209ES50/60209ES60/60209ES76 |
|
规格 |
1×1 mL/5×1 mL/10×1 mL/50×1 mL |
|
CAS号(CAS NO.) |
58-58-2 |
|
分子式(Molecular Fomular) |
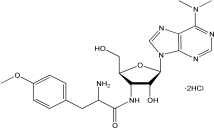
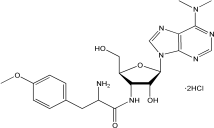
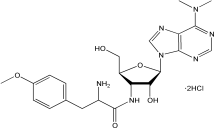
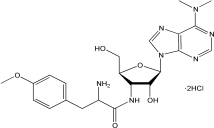
C22H29N7O5·2HCl |
|
分子量(Molecular Weight) |
544.43 g/mol |
|
纯度(Purity) |
≥98% |
|
外观(Appearance) |
溶液 |
|
浓度 (Concentration) |
10 mg/mL(溶于水) |
|
结构(Structure) |
|
组分信息
|
组分名称 |
60209ES10 |
60209ES50 |
60209ES60 |
60209ES76 |
|
规格 |
1×1 mL |
5×1 mL |
10×1 mL |
50×1 mL |
储存条件
-25~-15℃保存,有效期1年。
使用说明
1. 建议使用浓度
哺乳动物细胞:1~10 μg/mL,最佳浓度需要杀灭曲线来确定。推荐浓度,请看表1。
大肠杆菌:LB琼脂培养基筛选稳定转化pac基因的大肠杆菌,使用浓度为125 μg/mL。
【注】:使用嘌呤霉素筛选大肠杆菌稳转株需要精确的pH值调节,而且受宿主细胞本身的影响。
表1 嘌呤霉素盐酸盐的推荐浓度
|
细胞系 |
嘌呤霉素浓度 |
参考文献 |
|
B16(小鼠黑素细胞) |
1~2 μg/mL |
[1],[2] |
|
HEK293(人胚胎肾细胞) |
0.5~10 μg/mL |
[3] |
|
HeLa(人宫颈癌细胞) |
1~10 μg/mL |
[4],[5] |
|
MEF(小鼠成纤维细胞) |
1-5 μg/mL |
[4] |
|
HepG2(人肝细胞癌) |
0.5~5 μg/mL |
[6],[7] |
|
A549(肺癌细胞) |
1.5 μg/mL |
[8] |
|
人胚胎干 (ES) 细胞 |
0.5~5 μg/mL |
[9] |
- 嘌呤霉素杀灭曲线的确定(以shRNA转染或者慢病毒转导为例)
嘌呤霉素有效筛选浓度跟细胞类型、生长状态、细胞密度、细胞代谢情况及细胞所处细胞周期位置等有关。为了筛选到稳定表达的shRNA细胞株,确定杀死未转染/转导细胞的最低浓度嘌呤霉素至关重要。建议初次做实验的客户一定要建立适合自身实验体系的杀死曲线(kill curve)。
1)Day 1:24孔板内以5~8×104 cells/孔的密度铺板,铺足够量的孔,以确保后续的梯度实验的进行,37℃细胞孵育过夜。
2)Day 2:①准备筛选培养基:含不同浓度嘌呤霉素的新鲜培养基(如0~15 μg/mL,至少5个梯度);②往孵育过夜后的细胞内更换新鲜配制的筛选培养基;之后37℃孵育细胞。
3)Day 4:更换新鲜的筛选培养基,并观察细胞存活率。
4)根据细胞的生长状态,约2-3天更换新鲜的筛选培养基。
5)每日监测细胞,观察存活细胞率,确定抗生素筛选开始4~6天内有效杀死非转染或所有非转导细胞的药物最低浓度。
3. 哺乳动物稳定转染细胞株的筛选
等转染含有pac基因的质粒后,细胞在含有嘌呤霉素的培养基中增殖,以筛选出稳定转染子。
1)细胞转染48 h后,将细胞(原样或稀释)置于含有适当浓度嘌呤霉素的新鲜培养基中培养。
【注】:当细胞处于分裂活跃期时,抗生素作用最明显。细胞过于密集,抗生素产生的效力会明显下降。最好进行细胞分盘使其密度不超过25%。
2)每隔2~3天,移除和更换含有嘌呤霉素的培养基。
3)筛选7天后评估细胞形成的病灶。病灶可能需要额外的一周或者更多时间,这依赖于宿主细胞系和转染筛选效率。
【注】:每日进行细胞生长状态的观察。嘌呤霉素的筛选至少需要48 h,有效浓度嘌呤霉素的筛选周期一般在3-10天。
4)转移和放置5~10个抗性克隆到一个35 mm的培养皿中,用选择培养基继续培养7天。此次富集培养是为日后的细胞毒性实验做准备。
注意事项
1.嘌呤霉素为有毒化合物,操作时请小心拿放。
2.为了您的安全和健康,请穿实验服并戴一次性手套操作。
3.本产品仅用于科研用途,禁止用于人身上。
参考文献
[1] Furge KA. et al., 2001. Suppression of Ras-mediated tumorigenicity and metastasis through inhibition of the Met receptor tyrosine kinase. PNAS 98:10722-7.
[2] Díaz J. et al., 2014.Rab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasion.. J Cell Sci. 127:2401-6.
[3] Rössger K. et al., 2013. Reward-based hypertension control by a synthetic brain-dopamine interface. PNAS, 110:18150-5.
[4] Kamer I. et al., 2005. Proapoptotic BID Is an ATM effector in the DNA-damage response Cell. 122:593-603.
[5] Charnaux N. et al., 2005. RANTES (CCL5) induces a CCR5-dependent accelerated shedding of syndecan-1 (CD138) and syndecan-4 from HeLa cells and forms
[6] Gao J , Zhao N , Knutson M D , et al. The Hereditary Hemochromatosis Protein, HFE, Inhibits Iron Uptake via Down-regulation of Zip14 in HepG2 Cells[J]. Journal of Biological Chemistry, 2008, 283(31):21462-8.
[7] Huang J , Dibble C C , Matsuzaki M , et al. The TSC1-TSC2 complex is required for proper activation of mTOR2[J]. Molecular and Cellular Biology, 2008, 28(12):4104-4115.
[8] Nasser M W , Datta J , Nuovo G , et al. Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, Wang B, Suster S, Jacob ST, Ghoshal KDown-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem 283: 33394-33405[J]. Journal of Biological Chemistry, 2008, 283(48):33394-33405.
[9] Paatero A O , Hilkka T , Happonen L J , et al. Bacteriophage Mu integration in yeast and mammalian genomes[J]. Nucleic Acids Research, 2008, 36(22):e148-e148.
客户使用本产品发表的科研文献(部分)
[1] Zhang D, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022 May;24(5):766-782. doi: 10.1038/s41556-022-00894-z. Epub 2022 May 2. PMID: 35501370.
[2] Lu T, et al. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J Extracell Vesicles. 2022 May;11(5): e12218. doi: 10.1002/jev2.12218. PMID: 35524455; PMCID: PMC9077142.
[3] Chen S, et al. Identification of ubiquitin-specific protease 32 as an oncogene in glioblastoma and the underlying mechanisms. Sci Rep. 2022 Apr 19;12(1):6445. doi: 10.1038/s41598-022-09497-y. PMID: 35440702; PMCID: PMC9018837.
[4] Han L, et al. Uterus globulin associated protein 1 (UGRP1) binds podoplanin (PDPN) to promote a novel inflammation pathway during Streptococcus pneumoniae infection. Clin Transl Med. 2022 Jun;12(6): e850. doi: 10.1002/ctm2.850. PMID: 35652821; PMCID: PMC9161880.
[5] Sun Q, et al. MORTALIN-Ca2+ axis drives innate rituximab resistance in diffuse large B-cell lymphoma. Cancer Lett. 2022 Jul 1; 537:215678. doi: 10.1016/j.canlet.2022.215678. Epub 2022 Apr 18. PMID: 35447282.
嘌呤霉素(Puromycin)是由白黑链霉菌(Streptomyces alboniger)发酵代谢产生的一种氨基糖苷类抗生素,通过抑制蛋白质合成而杀死革兰氏阳性菌,各种动物和昆虫细胞。某种特殊情况下有效作用大肠杆菌。作用机制在于嘌呤霉素是氨酰-tRNA分子3´末端的类似物,能够与核糖体的A位点结合并掺入到延伸的肽链中。嘌呤霉素同A位点结合后,不会参与随后的任何反应,从而导致蛋白质合成的提前终止并释放出C-末端含有嘌呤霉素的不成熟多肽。
嘌呤霉素产生菌Streptomyces alboniger内发现的pac基因编码嘌呤霉素N-乙酰转移酶(PAC),赋予机体对嘌呤霉素产生抗性。这一特性如今普遍应用于筛选特定携带pac基因质粒的哺乳动物稳定转染细胞株。
嘌呤霉素在细胞稳转株筛选中的普遍应用与慢病毒载体的特性有关,现在商业化的慢病毒载体多数都携带pac基因。在某些特定情况下,嘌呤霉素亦可以用来筛选转化携带pac基因质粒的大肠杆菌菌株。
本品是无菌的、溶于蒸馏水的嘌呤霉素盐酸盐溶液,浓度为10 mg/mL(10 mg/mL in H2O),可直接用培养基或其他缓冲溶液稀释使用,适用于细胞培养,常用工作浓度为1~10 µg/mL。
产品信息
|
货号 |
60209ES10/60209ES50/60209ES60/60209ES76 |
|
规格 |
1×1 mL/5×1 mL/10×1 mL/50×1 mL |
|
CAS号(CAS NO.) |
58-58-2 |
|
分子式(Molecular Fomular) |
C22H29N7O5·2HCl |
|
分子量(Molecular Weight) |
544.43 g/mol |
|
纯度(Purity) |
≥98% |
|
外观(Appearance) |
溶液 |
|
浓度 (Concentration) |
10 mg/mL(溶于水) |
|
结构(Structure) |
|
组分信息
|
组分名称 |
60209ES10 |
60209ES50 |
60209ES60 |
60209ES76 |
|
规格 |
1×1 mL |
5×1 mL |
10×1 mL |
50×1 mL |
储存条件
-25~-15℃保存,有效期1年。
使用说明
1. 建议使用浓度
哺乳动物细胞:1~10 μg/mL,最佳浓度需要杀灭曲线来确定。推荐浓度,请看表1。
大肠杆菌:LB琼脂培养基筛选稳定转化pac基因的大肠杆菌,使用浓度为125 μg/mL。
【注】:使用嘌呤霉素筛选大肠杆菌稳转株需要精确的pH值调节,而且受宿主细胞本身的影响。
表1 嘌呤霉素盐酸盐的推荐浓度
|
细胞系 |
嘌呤霉素浓度 |
参考文献 |
|
B16(小鼠黑素细胞) |
1~2 μg/mL |
[1],[2] |
|
HEK293(人胚胎肾细胞) |
0.5~10 μg/mL |
[3] |
|
HeLa(人宫颈癌细胞) |
1~10 μg/mL |
[4],[5] |
|
MEF(小鼠成纤维细胞) |
1-5 μg/mL |
[4] |
|
HepG2(人肝细胞癌) |
0.5~5 μg/mL |
[6],[7] |
|
A549(肺癌细胞) |
1.5 μg/mL |
[8] |
|
人胚胎干 (ES) 细胞 |
0.5~5 μg/mL |
[9] |
- 嘌呤霉素杀灭曲线的确定(以shRNA转染或者慢病毒转导为例)
嘌呤霉素有效筛选浓度跟细胞类型、生长状态、细胞密度、细胞代谢情况及细胞所处细胞周期位置等有关。为了筛选到稳定表达的shRNA细胞株,确定杀死未转染/转导细胞的最低浓度嘌呤霉素至关重要。建议初次做实验的客户一定要建立适合自身实验体系的杀死曲线(kill curve)。
1)Day 1:24孔板内以5~8×104 cells/孔的密度铺板,铺足够量的孔,以确保后续的梯度实验的进行,37℃细胞孵育过夜。
2)Day 2:①准备筛选培养基:含不同浓度嘌呤霉素的新鲜培养基(如0~15 μg/mL,至少5个梯度);②往孵育过夜后的细胞内更换新鲜配制的筛选培养基;之后37℃孵育细胞。
3)Day 4:更换新鲜的筛选培养基,并观察细胞存活率。
4)根据细胞的生长状态,约2-3天更换新鲜的筛选培养基。
5)每日监测细胞,观察存活细胞率,确定抗生素筛选开始4~6天内有效杀死非转染或所有非转导细胞的药物最低浓度。
3. 哺乳动物稳定转染细胞株的筛选
等转染含有pac基因的质粒后,细胞在含有嘌呤霉素的培养基中增殖,以筛选出稳定转染子。
1)细胞转染48 h后,将细胞(原样或稀释)置于含有适当浓度嘌呤霉素的新鲜培养基中培养。
【注】:当细胞处于分裂活跃期时,抗生素作用最明显。细胞过于密集,抗生素产生的效力会明显下降。最好进行细胞分盘使其密度不超过25%。
2)每隔2~3天,移除和更换含有嘌呤霉素的培养基。
3)筛选7天后评估细胞形成的病灶。病灶可能需要额外的一周或者更多时间,这依赖于宿主细胞系和转染筛选效率。
【注】:每日进行细胞生长状态的观察。嘌呤霉素的筛选至少需要48 h,有效浓度嘌呤霉素的筛选周期一般在3-10天。
4)转移和放置5~10个抗性克隆到一个35 mm的培养皿中,用选择培养基继续培养7天。此次富集培养是为日后的细胞毒性实验做准备。
注意事项
1.嘌呤霉素为有毒化合物,操作时请小心拿放。
2.为了您的安全和健康,请穿实验服并戴一次性手套操作。
3.本产品仅用于科研用途,禁止用于人身上。
参考文献
[1] Furge KA. et al., 2001. Suppression of Ras-mediated tumorigenicity and metastasis through inhibition of the Met receptor tyrosine kinase. PNAS 98:10722-7.
[2] Díaz J. et al., 2014.Rab5 is required in metastatic cancer cells for Caveolin-1-enhanced Rac1 activation, migration and invasion.. J Cell Sci. 127:2401-6.
[3] Rössger K. et al., 2013. Reward-based hypertension control by a synthetic brain-dopamine interface. PNAS, 110:18150-5.
[4] Kamer I. et al., 2005. Proapoptotic BID Is an ATM effector in the DNA-damage response Cell. 122:593-603.
[5] Charnaux N. et al., 2005. RANTES (CCL5) induces a CCR5-dependent accelerated shedding of syndecan-1 (CD138) and syndecan-4 from HeLa cells and forms
[6] Gao J , Zhao N , Knutson M D , et al. The Hereditary Hemochromatosis Protein, HFE, Inhibits Iron Uptake via Down-regulation of Zip14 in HepG2 Cells[J]. Journal of Biological Chemistry, 2008, 283(31):21462-8.
[7] Huang J , Dibble C C , Matsuzaki M , et al. The TSC1-TSC2 complex is required for proper activation of mTOR2[J]. Molecular and Cellular Biology, 2008, 28(12):4104-4115.
[8] Nasser M W , Datta J , Nuovo G , et al. Nasser MW, Datta J, Nuovo G, Kutay H, Motiwala T, Majumder S, Wang B, Suster S, Jacob ST, Ghoshal KDown-regulation of micro-RNA-1 (miR-1) in lung cancer. Suppression of tumorigenic property of lung cancer cells and their sensitization to doxorubicin-induced apoptosis by miR-1. J Biol Chem 283: 33394-33405[J]. Journal of Biological Chemistry, 2008, 283(48):33394-33405.
[9] Paatero A O , Hilkka T , Happonen L J , et al. Bacteriophage Mu integration in yeast and mammalian genomes[J]. Nucleic Acids Research, 2008, 36(22):e148-e148.
客户使用本产品发表的科研文献(部分)
[1] Zhang D, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022 May;24(5):766-782. doi: 10.1038/s41556-022-00894-z. Epub 2022 May 2. PMID: 35501370.
[2] Lu T, et al. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J Extracell Vesicles. 2022 May;11(5): e12218. doi: 10.1002/jev2.12218. PMID: 35524455; PMCID: PMC9077142.
[3] Chen S, et al. Identification of ubiquitin-specific protease 32 as an oncogene in glioblastoma and the underlying mechanisms. Sci Rep. 2022 Apr 19;12(1):6445. doi: 10.1038/s41598-022-09497-y. PMID: 35440702; PMCID: PMC9018837.
[4] Han L, et al. Uterus globulin associated protein 1 (UGRP1) binds podoplanin (PDPN) to promote a novel inflammation pathway during Streptococcus pneumoniae infection. Clin Transl Med. 2022 Jun;12(6): e850. doi: 10.1002/ctm2.850. PMID: 35652821; PMCID: PMC9161880.
[5] Sun Q, et al. MORTALIN-Ca2+ axis drives innate rituximab resistance in diffuse large B-cell lymphoma. Cancer Lett. 2022 Jul 1; 537:215678. doi: 10.1016/j.canlet.2022.215678. Epub 2022 Apr 18. PMID: 35447282.
Q: 60209,T细胞阳性细胞的筛选浓度。还是按照细胞培养的浓度来的吗?
A: 说明书中有推荐的浓度,但是只是一个参考范围,具体还是要测试一下。说明书表格中没有T细胞,但是可以参考常规的浓度范围:1~10 µg/mL。
[1] Zhang D, Liu Y, Zhu Y, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022;24(5):766-782. doi:10.1038/s41556-022-00894-z(IF:28.824)
[2] Lu T, Zhang Z, Zhang J, et al. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J Extracell Vesicles. 2022;11(5):e12218. doi:10.1002/jev2.12218(IF:25.841)
[3] Zhou R, Wu Q, Wang M, et al. The protein phosphatase PPM1A dephosphorylates and activates YAP to govern mammalian intestinal and liver regeneration. PLoS Biol. 2021;19(2):e3001122. Published 2021 Feb 25. doi:10.1371/journal.pbio.3001122(IF:8.029)
[4] Sun J, Guo Y, Fan Y, Wang Q, Zhang Q, Lai D. Decreased expression of IDH1 by chronic unpredictable stress suppresses proliferation and accelerates senescence of granulosa cells through ROS activated MAPK signaling pathways. Free Radic Biol Med. 2021;169:122-136. doi:10.1016/j.freeradbiomed.2021.04.016(IF:7.376)
[5] Xu D, Jiang S, He Y, Jin X, Zhao G, Wang B. Development of a therapeutic vaccine targeting Merkel cell polyomavirus capsid protein VP1 against Merkel cell carcinoma. NPJ Vaccines. 2021;6(1):119. Published 2021 Oct 5. doi:10.1038/s41541-021-00382-9(IF:7.344)
[6] Zhou YM, Yang YY, Jing YX, et al. BMP9 Reduces Bone Loss in Ovariectomized Mice by Dual Regulation of Bone Remodeling. J Bone Miner Res. 2020;35(5):978-993. doi:10.1002/jbmr.3957(IF:5.854)
[7] Zhou L, Chen W, Cao C, et al. Design and synthesis of α-naphthoflavone chimera derivatives able to eliminate cytochrome P450 (CYP)1B1-mediated drug resistance via targeted CYP1B1 degradation. Eur J Med Chem. 2020;189:112028. doi:10.1016/j.ejmech.2019.112028(IF:5.573)
[8] Chen P, Wang S, Cao C, et al. α-naphthoflavone-derived cytochrome P450 (CYP)1B1 degraders specific for sensitizing CYP1B1-mediated drug resistance to prostate cancer DU145: Structure activity relationship. Bioorg Chem. 2021;116:105295. doi:10.1016/j.bioorg.2021.105295(IF:5.275)
[9] Yan YL, Huang ZN, Zhu Z, et al. Downregulation of TET1 Promotes Bladder Cancer Cell Proliferation and Invasion by Reducing DNA Hydroxymethylation of AJAP1. Front Oncol. 2020;10:667. Published 2020 May 21. doi:10.3389/fonc.2020.00667(IF:4.848)
[10] Huang H, Zou X, Zhong L, et al. CRISPR/dCas9-mediated activation of multiple endogenous target genes directly converts human foreskin fibroblasts into Leydig-like cells. J Cell Mol Med. 2019;23(9):6072-6084. doi:10.1111/jcmm.14470(IF:4.658)
[11] Yang R, Yang E, Shen L, Modlin RL, Shen H, Chen ZW. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth [published correction appears in J Immunol. 2019 Oct 1;203(7):2020]. J Immunol. 2018;200(7):2405-2417. doi:10.4049/jimmunol.1701073(IF:4.539)
[12] Huang H, Zhong L, Zhou J, et al. Leydig-like cells derived from reprogrammed human foreskin fibroblasts by CRISPR/dCas9 increase the level of serum testosterone in castrated male rats. J Cell Mol Med. 2020;24(7):3971-3981. doi:10.1111/jcmm.15018(IF:4.486)
[13] Chen S, Chen X, Li Z, et al. Identification of ubiquitin-specific protease 32 as an oncogene in glioblastoma and the underlying mechanisms. Sci Rep. 2022;12(1):6445. Published 2022 Apr 19. doi:10.1038/s41598-022-09497-y(IF:4.380)
[14] Fang X, Liu Y, Xiao W, et al. Prognostic SLC family genes promote cell proliferation, migration, and invasion in hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai). 2021;53(8):1065-1075. doi:10.1093/abbs/gmab076(IF:3.848)
[15] Liu X, Tao J, Yao Y, et al. Resveratrol induces proliferation in preosteoblast cell MC3T3-E1 via GATA-1 activating autophagy. Acta Biochim Biophys Sin (Shanghai). 2021;53(11):1495-1504. doi:10.1093/abbs/gmab135(IF:3.848)
[16] Jin Y, Qin X. Paired like homeodomain 1 and SAM and SH3 domain-containing 1 in the progression and prognosis of head and neck squamous cell carcinoma. Int J Biochem Cell Biol. 2020;127:105846. doi:10.1016/j.biocel.2020.105846(IF:3.673)
[17] Ge L, Tan W, Li G, Gong N, Zhou L. Circ_0026134 promotes NSCLC progression by the miR-3619-5p/CHAF1B axis. Thorac Cancer. 2022;13(4):582-592. doi:10.1111/1759-7714.14301(IF:3.500)
[18] Zhu X, Li M, Jia X, et al. The homeoprotein Msx1 cooperates with Pkn1 to prevent terminal differentiation in myogenic precursor cells. Biochimie. 2019;162:55-65. doi:10.1016/j.biochi.2019.04.003(IF:3.362)
[19] Hu C, Dai J, Lin X, Meng Y, Liang H. Effect of RSK4 on Biological Characteristics of Gastric Cancer. Cancer Manag Res. 2020;12:611-619. Published 2020 Jan 28. doi:10.2147/CMAR.S238132(IF:2.886)
[20] Liu SL, Zhou YM, Tang DB, et al. LGR6 promotes osteogenesis by activating the Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2019;519(1):1-7. doi:10.1016/j.bbrc.2019.08.122(IF:2.705)
[21] Lai Y, Zhang Z, Li J, et al. STYK1/NOK correlates with ferroptosis in non-small cell lung carcinoma. Biochem Biophys Res Commun. 2019;519(4):659-666. doi:10.1016/j.bbrc.2019.09.032(IF:2.705)
[22] Li K, Xu J, Xue K, et al. Deficiency of two-pore segment channel 2 contributes to systemic lupus erythematosus via regulation of apoptosis and cell cycle. Chin Med J (Engl). 2022;135(4):447-455. Published 2022 Jan 12. doi:10.1097/CM9.0000000000001893(IF:2.628)
[23] Yang J, Tang Y, Chen W, et al. Establishment and characterization of an immortalized human chondrocyte cell line. Biotechnol Lett. 2020;42(5):707-716. doi:10.1007/s10529-020-02827-y(IF:1.977)
[24] Xiao L, Xue Y, Zhang C, Wang L, Lin Y, Pan G. The involvement of multidrug and toxin extrusion protein 1 in the distribution and excretion of berberine. Xenobiotica. 2018;48(3):314-323. doi:10.1080/00498254.2017.1300707(IF:1.932)
[25] Chen W, Liu Z, Mai W, Xiao Y, You X, Qin L. FZD8 Indicates a Poor Prognosis and Promotes Gastric Cancer Invasion and Metastasis via B-Catenin Signaling Pathway. Ann Clin Lab Sci. 2020;50(1):13-23. (IF:1.256)
[26] Zhang J, Zhang Z. Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression. Open Life Sci. 2021;16(1):495-510. Published 2021 May 22. doi:10.1515/biol-2021-0047(IF:0.938)